quality
ac.biomed is highly specialized in tailoring the best testing for your application!
We can cover almost all cardiovascular devices where fluid interaction is vital.

ac.biomed provides quality services.
Therefore, we have implemented our Quality Management to international standards.
Our standard laboratory testing and service documentation are certified to ISO 9001 and compliant to ISO 17025. We have been approved regularly through independent audits. Our quality processes are standardized so we can use our energy to serve your project.
Quality Methods
Our testing programs are designed to cover all international standards. Our heart valve testing is designed to meet all of ISO 5840 and current FDA heart valve requirements. We offer testing methods for oxygenators, blood pumps and other devices in compliance with international standards and guidelines. Our documentation meets the highest international requirements and is regularly submitted for Notified Bodies and international regulatory agencies such as the US FDA. Our test methods and our robust test equipment are validated from the very beginning. When we develop new methods for our clients, we provide the necessary background information the client needs.
Quality People
Our team is comprised mostly of university-educated biomedical engineers. All of us have worked in industry projects and scientific environments. There is a clear commitment to the quality of our work, from every one of us, every day. Members of our team are part of ISO and IEC international standardization committees.
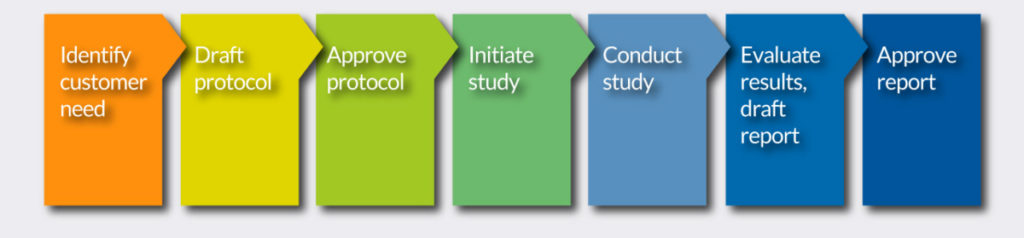
Our Workflow
service around the world
Our clients are mainly medical device manufacturers from all over the world. They range from audacious startups to global players and market leaders with long and successful histories. ac.biomed has the processes to serve your needs.
contact us
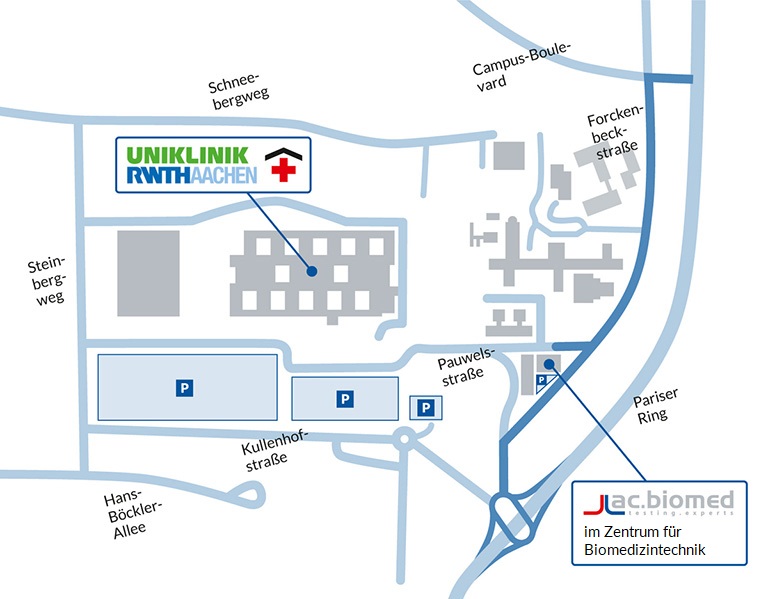
how to find us
Follow the Vaalser Straße and turn right on Pariser Ring, immediately get in the right lane (exit ‘Uniklinik’). In the roundabout take the third exit ‘Forckenbeckstrasse’. You can access the Pauwelsstrasse from the first and second street on the left hand side. The Zentrum für Biomedizintechnik (ZBMT) is situated in building no. 17 and so is ac.biomed.