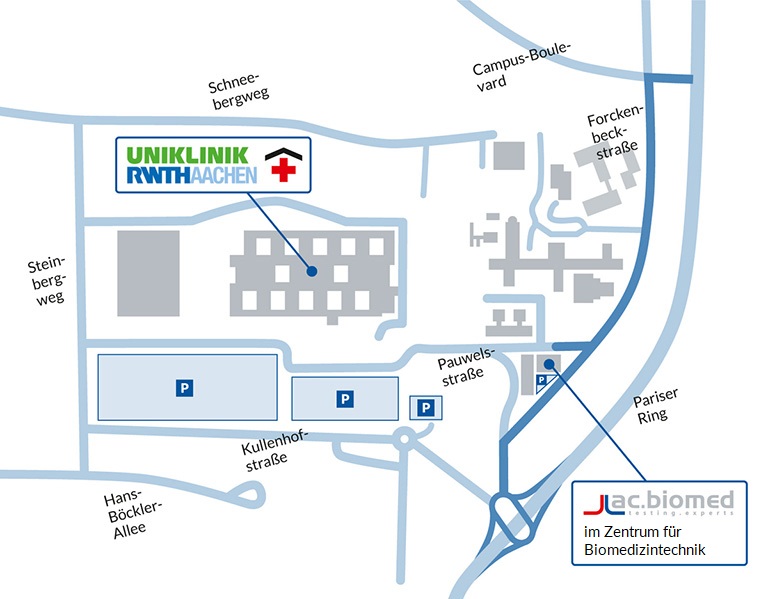
methods
Standardized testing and individual setups. ac.biomed provides objective feedback on your products’ safety, effectiveness and performance. Our clients profit from our know-how. Use ac.biomed as your partner for independent assessment and improvement of your innovative products.

Patients must rely on heart valves for a lifetime. Are your products safe and effective over such an extend period? ac.biomed offers complete accelerated wear tests (durability) according to ISO 5840 and FDA guidelines for development and regulatory approval of heart valve prostheses.
The tests:
- we are the only tester worldwide with 12 independent test chambers
- conform to international standards (ISO and FDA)
- adaptable for every prosthesis design and anatomical boundary condition
- biological, mechanical and other artificial prostheses
- variable test setups for oval annuli testing, valve-in-valve studies etc.
- self-developed and validated test systems and methods
Projects:
- rapid feasibility studies for R&D projects
- complete approval studies for submission to regulatory agencies, e.g. FDA
- individual studies tailored to your needs
- high volume production sample test
Evaluation & documentation:
- standard protocol and report ready for regulatory approval submission
- complete documentation corresponding to regulatory requirements
- protocol according to customer specifications
- documentation including high speed video if needed
Customer support:
We offer established standard testing methods and individual tests developed to your need. For performance evaluation, product benchmarking or approval studies: Let ac.biomed develop the best testing strategy for you!

We evaluate the hydrodynamic performance of heart valve prostheses according to ISO 5840 and FDA guidelines. With our circulatory mock loop, we are able to apply standard and customized physiological pressures and flow conditions to your valve.
The tests:
- Testing in stationary and pulsatile flow
- Bernoulli verification
- Effective orifice area (EOA)
- Pressure gradient
- Regurgitation
- Valve closing mechanics, leaflet coaptation
- Variable test setups for ovality testing, valve-in-valve studies etc.
- Cavitation test
- Migration study (valve in valve)
Projects:
- rapid feasibility studies for R&D projects
- complete approval studies for submission to regulatory agencies or Notified Bodies, e.g. FDA
- individual tests according to customer demand (e.g. anatomy-specific studies)
Evaluation & documentation:
- standard protocol and report ready for regulatory approval submission
- complete documentation corresponding to regulatory requirements
- protocol according to customer specifications
- documentation including high speed video if needed
Customer support
Regulatory approval studies are standard. You need more? We provide individual testing setups in our circulatory mock loops. Our variable and modular system is built to realize almost all meaningful test setups. For further information, contact us!
In vivo performance is complex to assess in medical devices. Learn more about your product before going live! ac.biomed provides numerical, mock & simulated use loops. Test setups are high value and time-saving for your development. Use our comprehensive circulatory simulation system to assess your device’s performance.
The tests:
- permeability and compliance testing
- Real geometry implantation simulation
- Transparent test setups for flow visualization and measurement
- Interaction between circulatory system and implant
- Numerical simulation
Projects:
- Implant performance testing
- Testing and development of clinical implantation procedures
- Placement studies
- Computational flow dynamics
Evaluation & documentation:
- standard protocol and report under full ISO quality control
- complete documentation corresponding to regulatory requirements
- protocol according to customer specifications
- documentation including high speed video if needed
- scientific assessment of results
Customer support:
Your device is innovative! Do you need to assess performance? We support our customers with individual test setups. ac.biomed supports customers throughout the development process of their products.
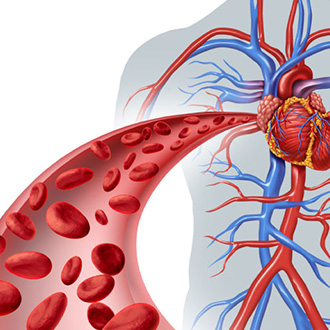
Blood compatibility is one of the key issues in developing implants with blood contact. ac.biomed offers a large range of studies to evaluate the thrombogenic and hemolytic potential of medical devices, including hemolysis tests for blood pumps according to ASTM F1841.
The tests:
- Blood damage in rotary blood pumps according to ASTM F1841
- Blood damage in pulsatile pumps, stent grafts and systems
- Material and surface evaluation (e.g. Chandler-loop tests)
- Pumped circuits with individual test chambers
- Activation of extrinsic and intrinsic coagulation effect
Projects:
- Regulatory approval tests for blood pumps according to ASTM F1841
- Comparison of different materials, geometries or systems
- Benchmark-tests to other marketed devices
- Design and performance testing during development
Evaluation & documentation:
- standard protocol and report ready for regulatory approval submission
- complete documentation corresponding to regulatory requirements
- protocol according to customer specifications
- documentation including high speed video if needed
- scientific assessment of results
Customer support:
Standard tests give standard answers. Need more? For development, regulatory approval or individual needs, such as comparing different products, ac.biomed is your partner for evaluation of hemolysis and thrombogenic potential for any product.
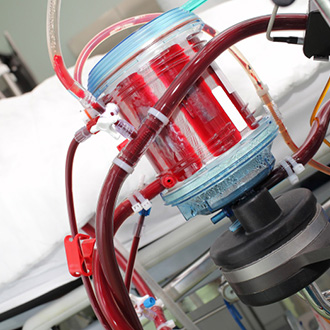
The clinical performance of oxygenation devices is vital. Gas exchange and hemolysis are standardized tests for oxygenators. ac.biomed offers the complete test setup for oxygenators according to ISO 7199 and FDA guidance for cardiopulmonary bypass.
The tests:
- Hemolysis
- Gas exchange performance
- Pressure drop
- Tubing tests
- Operation qualification
Projects:
- feasibility studies for R&D projects
- complete approval studies for submission to regulatory agencies, e.g. FDA
- Qualification and verification of different models and geometries
- individual tests according to customer requests
- comparative benchmark tests
Evaluation & documentation:
- standard protocol and report ready for regulatory approval submission
- complete documentation corresponding to regulatory requirements
- protocol according to customer specifications
- documentation including high speed video if needed
- scientific assessment of results
Customer support:
ac.biomed offers experimental setups, in addition to development evaluation and approval-relevant tests according to established guidelines. As a competent partner, we support our customers throughout the complete product development.
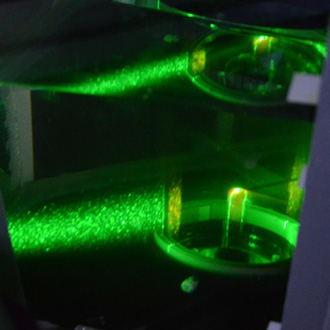
In vivo flow dynamics are complex. Learn more about your cardiovascular implants’ blood flow characteristics! ac.biomed uses various techniques such as PIV to assess the flow field of a device.
The tests:
- Flow through heart valve prostheses
- Transparent test setups for flow visualization and measurement
- Estimation of shear stresses
- Particle Image Velocimetry (PIV) or classical light scatter
- High speed camera evaluation
- Numerical simulation (with our partner enmodes)
Projects:
- Flow field analysis and hydrodynamic evaluation
- Assessment of clinically relevant flow phenomena
- Computational flow dynamics
Evaluation & documentation:
- standard protocol and report under full ISO quality control
- complete documentation corresponding to regulatory requirements
- protocol according to customer specifications
- documentation, including video if needed
- scientific assessment of results
Customer support:
Your device is innovative! Do you need to assess its performance? We support our customers with individual test setups. ac.biomed supports customers throughout the development process of their products.

Haven’t found what you were looking for? No worries! ac.biomed develops individualized test methods and testers for medical devices. We implement, validate, and apply standardized tests. All of our test methods are scientifically validated. We support our customers with intelligent solutions for virtual anatomy studies, self-manufactured silicon vessel models, the development of complex test setups and more.
service around the world
Our clients are mainly medical device manufacturers from all over the world. They range from audacious startups to global players and market leaders with long and successful histories. ac.biomed has the processes to serve your needs.
contact us
how to find us
Follow the Vaalser Straße and turn right on Pariser Ring, immediately get in the right lane (exit ‘Uniklinik’). In the roundabout take the third exit ‘Forckenbeckstrasse’. You can access the Pauwelsstrasse from the first and second street on the left hand side. The Zentrum für Biomedizintechnik (ZBMT) is situated in building no. 17 and so is ac.biomed.