service
With our vast experience we support our customers from the first laboratory samples of their products up to clinical approval and even beyond. Members of our team serve on the international standardization committee for ISO 5840 on heart valve prostheses.
Regulatory testing
ac.biomed conducts testing according to international standards such as ISO 5840. Such studies are of high value for our clients, as they form a vital component of their regulatory approval documentation. Regulatory testing is conducted under our rigorous quality system that conforms to international standards.
For your safety!
Development support
ac.biomed supports our clients during their development phase. From doing quick R&D primary evaluation to in-depth evaluation of laboratory samples, we follow your development process. If you are new to the field, we help to evaluate the most beneficial approach with regards to efficiency, efficacy, and scientific validity.
Tell us about your approach!

Development of test methods and devices
Your device is novel and highly innovative. Is there no standard yet to follow? We develop, according to your needs, new testing methods that provide evidence of your device’s characteristics or function. Or both. If you want, we can even develop new test equipment that you can use in your own lab, after we have qualified with a preliminary study.

service around the world
Our clients are mainly medical device manufacturers from all over the world. They range from audacious startups to global players and market leaders with long and successful histories. ac.biomed has the processes to serve your needs.
contact us
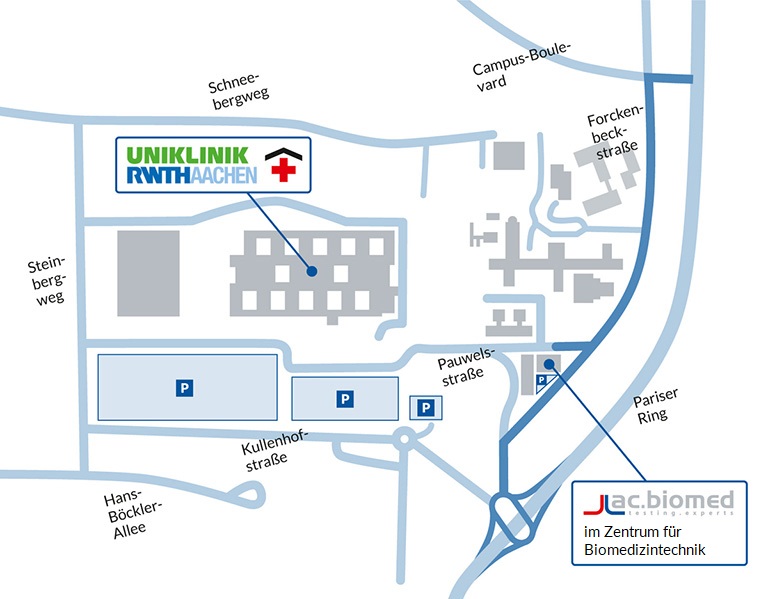
how to find us
Follow the Vaalser Straße and turn right on Pariser Ring, immediately get in the right lane (exit ‘Uniklinik’). In the roundabout take the third exit ‘Forckenbeckstrasse’. You can access the Pauwelsstrasse from the first and second street on the left hand side. The Zentrum für Biomedizintechnik (ZBMT) is situated in building no. 17 and so is ac.biomed.